Product description
SANOMAG® uses the power of natural magnetism to promote well-being and regeneration. This certified medical device generates a strong static magnetic field used in pain, stress, sleep disorders and muscle tension – proven for over 40 years in medicine and self-application.
With its permanent magnets, SANOMAG generates a static magnetic field that can balance out disturbances such as those caused by mobile phones, power lines or geological conditions, helping create a harmonious atmosphere.
SANOMAG® is a permanent magnetic field therapy device that generates a static magnetic field with a maximum magnetic flux density of around 100 mT (equivalent to 1000 Gauss).
This field can be used to improve general well-being and strengthen the immune system in humans and animals, as well as for complementary medical support in wound healing, treatment of tension, tired legs, colds, migraines, insect bites, bruises, burns, various types of pain, and selected eye and lung conditions.
SANOMAG is suitable for self-treatment or use by doctors, veterinarians, physiotherapists, and alternative practitioners.
SANOMAG is registered in the medical device register with the Basic UDI-DI (GMN): 91200576810397. The UDI-DI of the product is: 09120057681036. For details on indications, see the brochure “SANOMAG-GRANDER”.
Significant risks exist for people and animals with implanted electronic or magnetic devices (e.g., pacemakers, defibrillators, insulin pumps, chips, hearing aids, magnet-based joints).
Safe functioning of such devices is generally guaranteed only up to a magnetic field strength of 100 µT (1 Gauss). SANOMAG generates up to 25 mT (250 Gauss) at its body and 170 mT (1700 Gauss) at its handles.
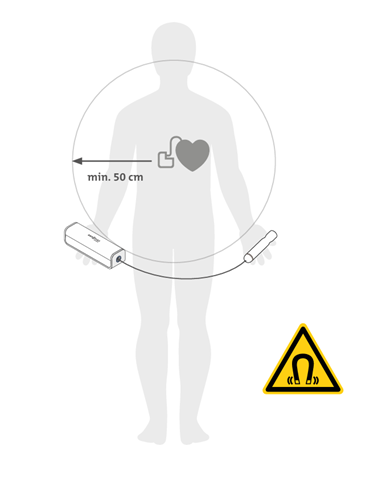
Therefore, a distance of at least 50 cm from the SANOMAG must be maintained unless explicitly approved by the implant manufacturer.
The same applies to external devices such as hearing aids or insulin pumps. These must be removed or kept at a safe distance, unless permission is granted by the manufacturer.
The same also applies to magnetic storage media, PCs and similar electronic devices.
For users without electronic or magnetic devices, no risks are currently known.
According to ICNIRP Guidelines (Health Physics 96(4):504-514; 2009), even continuous exposure to 400 mT (4000 Gauss) is considered safe.
However, SANOMAG may pose serious risk to users with such devices. Refer to the safety section for risk minimisation strategies.
ICNIRP is the International Commission on Non-Ionizing Radiation Protection.
A general description of the main function of SANOMAG® is found in patent EP 3 011 996 B1.
SANOMAG® is a registered trademark. SRN AT-MF-000012305
GRANDER GmbH,
Bergwerksweg 10,
A-6373 Jochberg,
Heribert Grander – Management SANOMAG®
We hereby declare that GRANDER GmbH is solely responsible for this EU Declaration of Conformity.
Basic UDI-DI: 912 00 576 810 397
Product: Magnetic field therapy device SANOMAG® – Risk class: I
This product complies with Regulation (EU) 2017/745. No further specifications apply.
The European Patent No. 3 011 996 applies to the product covered by this declaration.